Lateral flow assay reader development
Abingdon Health Invited Gm Design Development to design their new rapid lateral flow assay reader – the ADxLR5®. The lateral flow test is used for quantitation measurement of kappa (K) and lambda (?) immunoglobulin free light chains (FLC) in serum.
Challenges
Several key challenges existed, and one was the time to market required. To reduce the development and validation lead-times, Gm Design Development opted to use an all-in-one large 10.1” capacitive touch screen with integrated PC module, simplifying this aspect of the software development and validation, as well as the electronics themselves. The product was both CE marked, and FDA registered. Designed to meet regulatory requirements, so it could be used in different industry sectors.
GUI development
We helped to develop the test protocol, producing an intuitive and clear GUI (Graphic User Interface) allowing users to operate the reader easily, inputting data whilst wearing latex gloves. The step by step operating system ensured clinicians needed little training.
Drawer system
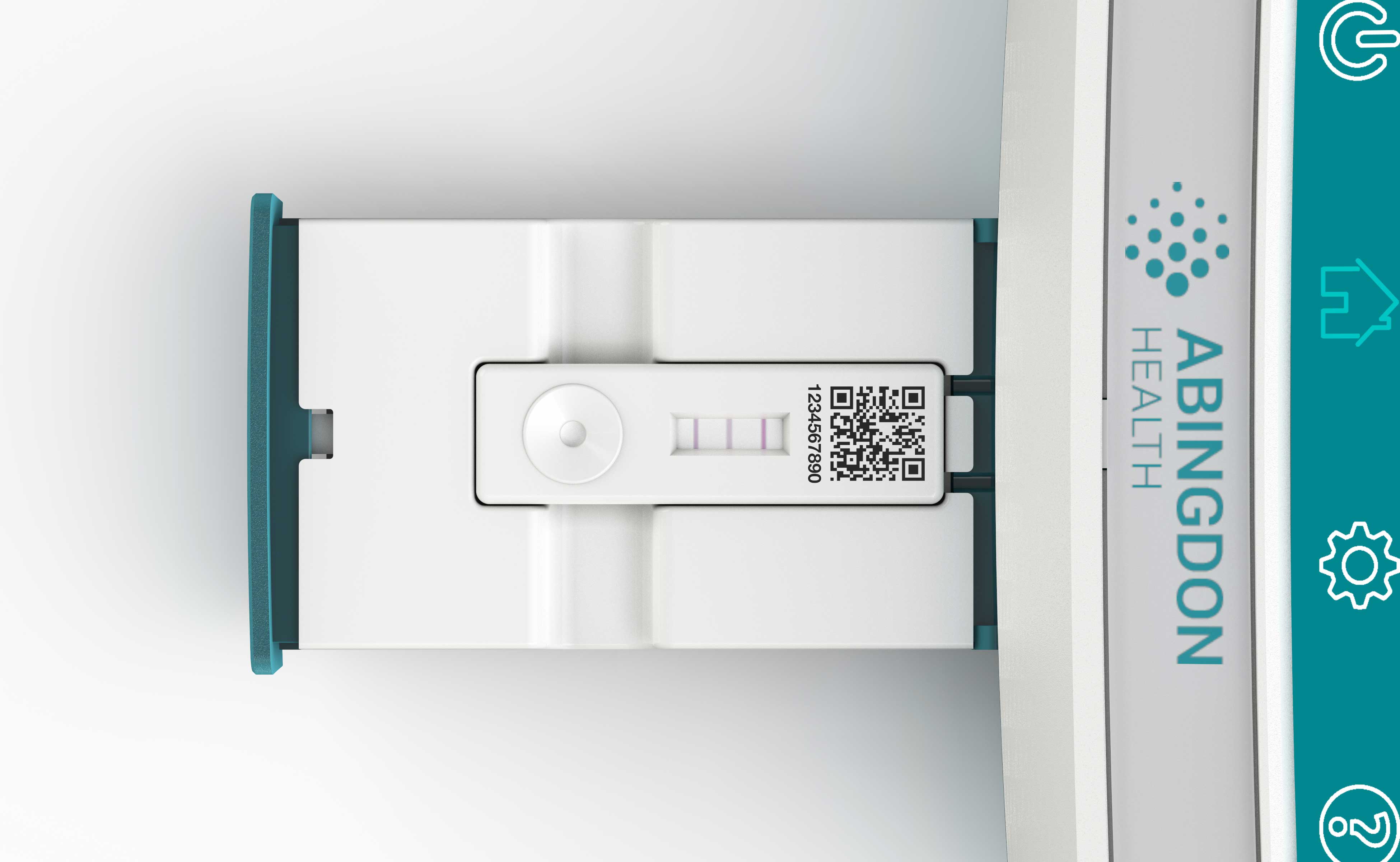
We developed a soft-eject draw feature using a constant force spring to provide the ejection force and a damper mechanism to deliver the soft-eject. The drawer accommodates both single lateral flow assays as well as wider multiplexed lateral flow assays. By simply changing an insert moulding, a wider recess in the drawer is achieved.
The position of the assay being read by the optics was also critical, so we designed an integrated spring mechanism within the drawer assembly which guaranteed that the assay was always pushed hard up against a stop within the reader time after time.
Data transfer
Data transfer was developed into the reader via WiFi, ethernet or USB. A barcode scanner was included to help make the test protocol secure.
Manufacture
We designed the reader in under six months, working closely with the toolmaker. We worked with the toolmaker to agree the tool design